|
Inclusion Pigments - Andreas Widhalm January 25, 2008 |
Zinc Silicate Crystalline Glaze Pottery A chronicle of my recent progress and a way for me to keep it straight in my head! |
Inclusion Pigments
(aka Encapsulated Stain)
Written to John Tilton by Andreas Widhalm, December, 2007 and edited by me. Plus further dialogue.
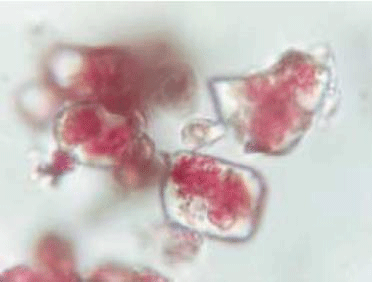
This photo clearly shows what is meant by the phrase "inclusion pigment":

You can see the red cadmium-selenium-sulfide particles enclosed in colourless, transparent zirconium silicate crystal.
Cd-S-Se
is thermally stable up to 900°C in normal atmospheres.
Beyond
that
the pigment decomposes to
form
CdO,
SO2, SO3, and SeO2, and loses its colour and
pigment quality.
As
long as the Cd-S-Se
particles are embedded in the ZrSiO4 crystal they are protected
against oxidation and the chemical attack of the glaze frit or flux.
There are 2 possibilities to break the crystal and destroy the colour:
1. physical attack of the particles through excessive milling.
The
first point is easy to avoid.
Don’t mill the glaze too long. 30 minutes is
OK,
but that’s the border.
The
second point shows the tragedy itself. Crystalline glazes are of high alkali
nature. There are only some metal oxides that help the pigment survive or
improve its brightness.
The
good boys are: Lead, Calcium, Magnesium, Boron and Zircon.
The neutrals are Zinc, Barium and Alumina.
The bad boys are: Sodium, Potassium, Lithium, Fluorine.
So Its clear that you cannot put the pigment into the glaze, because it a real unfriendly environment. During the heat up of the glaze in the kiln the first ingredient that melts is the frit. Compositions like 3110 or 90208 and all the others are the nightmare of all inclusion pigments. In the later stages of the firing zinc oxide dissolves in the frit but comes too late to offer healing hands for the pigment.
There is only one small gap in this chain of impossibilities
- put
the pigment in a slip where it is protected
from
the killer alkalis. The pigment is attacked in the very first stage of the
firing by the frit, but in the same moment the frit starts to dissolve the slip.
So early enough for the rest of the pigment an intermediate layer between the
slip and the glaze forms which is rich in alumina and silica with a very high
viscosity on the slip side.
This,
in
most cases,
is the last resort for the pigment. And as we all know, or better as we all have
seen with
Denis (Caraty's)
work, it works if all is going right.
I
personally haven’t yet
tried this “red slip under crystalline glaze” system. I have done hundreds of
test in the industry,
mainly
with glazes,
but also with slips.
If the pigment is
attacked
by
the glaze
too
much
zinc
oxide and zircon silicate
additions
improve
its survival.
Use
it additionally in the slip from 5-20% if necessary.
I do not know how deep you want to go into technical details, but if you want to go deeper, tell me.
Hope it helps a bit.
Andreas
Additional Info.......
Dear Phil!
Thanks’ for your mail, and the amiability to correct what I had written to John! You have done it excellent!
You are right, I am European. I live in a small village close to Vienna in Austria. I am a potter, with a strong technical background because I work for a glaze and pigment company here since 26 years. My heart belongs to my studio where I have my potters wheel and do some stone ware and porcelain with crystalline glazes.
I am a guest on you website almost daily since a long time, and I really enjoy it!
To your questions:
1., The cadmium-sulphide particles are embedded in transparent zirconium-silicate crystals. Glass as the matrix would not survive in the glaze. We have tried it but with no success. The pigments are produced at a temperature of about 900°C using zirconium oxide, silica, and some additives plus the cadmium pigments, to promote the crystal formation at that low temperature. In the moment the crystals constitute the cadmium pigments are included in the crystalline matrix of the single zirconium silicate particles. It sounds easy, but in practice it’s a difficult production process and a hard work to keep the quality constant.
2., It is common in the ceramic industry to add substances into a slip which improves the colour of a stain or oxide, or simply for stabilising reasons. Nowadays we use more non-plastic materials in slips than ever before. Especially zirconium silicate is used up to 50% and sometimes more to get a strong white base with a low shrinkage plus the benefit that no other raw material can stabilize the zirconium pigments more than it. This is valid for the whole group of zirconium stains: Zr-Si-V, Zr-Si-Pr, Zr-Si-Fe, Zr-Si-C, Zr-Si-Au, and Zr-Si-Cd-S-Se.
3., Of course, these additives have a strong impact on the nucleation rate of the glaze, but not as much as the pigments itself! The reason is that zinc oxide or zirconium silicate is much smaller in particle size than the pigments. The main problem concerning the nucleation rate is the fact that the pigments are so coarse that their peaks are reaching the glaze surface. This is an unintentional “seed crystal”, but difficult to avoid. We are talking about a pigment size of about 80 – 120 µm!
The zinc oxide and the zirconium silicate is not a problem, you only have to adjust the glaze to the more “refractory” under layer. (More frit, less zinc oxide, less silica)
4, It is necessary to adjust the glaze for each pigment or pigment combination. What makes the game even more complicated are the pigment composition variances from batch to batch.
The stains are products with a given formulation, fired to a fixed temperature, and milled. A classic solid state reaction. The industry is very careful in holding all the parameters constant. But in most cases the pigments come out of this process from batch to batch with changing colour or better colour strength. Therefore it is common and necessary for pigment companies to correct a lot of batches. This is done with similar substances of the stains main composition. In case of inclusion pigments this could be silica or zirconium silicate. We are talking about additions of 0 – 25%! So if you want to work with pigments under crystalline glazes it would be a wise decision to buy a bigger amount of pigment and do the glaze adjustment work. Otherwise it could be a never running system when you buy a new batch of pigment witch is not or heavily corrected by the manufacturer.
I hope this will help a little bit, and if you have additional questions please do not hesitate to ask me!
Yours truly,
Andreas
PS:
I hope I can arrange some tests this week with slips and stains under crystalline glazes to bring more light into this dark field of ceramic possibilities.
Von:
Phil Hamling [mailto:pdah@optonline.net]
Gesendet: Samstag, 26. Januar
2008 18:54
An: andreas.widhalm@inode.at
Cc: _John
Tilton
Betreff: [?? Probable Spam] FW:
AW: Encapsulated Stains
Andreas,
Thanks for allowing me to add the information you supplied to John regarding inclusion pigments to my web site.
When I saw the photo many long dormant brain cells from my days at Alfred University woke up. I found the technical discussion very informative.
As far as your English goes I concur with John. I think it is excellent, and much better than that of some people who use it here as their native language. I edited some of your words, but tried not to make it read as if it were written by me. How did I do?
You seem to have a certain American colloquial style to you English. I'm guessing you are not an American, but don't know where you are on the planet. Are you European?
Perhaps you can answer a few questions I have, including:
1) Are the red cadmium-selenium-sulfide particles actually enclosed in colourless, transparent zirconium silicate CRYSTAL or is it a GLASS?
2) Is the addition of zinc oxide or zircon silicate to the slip a common practice or is it mostly used without these additions?
3) Does the addition of zinc oxide or zircon silicate to the slip have a significant impact on nucleation rate?
4) Is it common to adjust the composition of the glaze for each inclusion pigment or pigment combination? I started working with 3 Cerdec Degussa inclusion pigments. Yellow, Red and Brilliant Red (I have the numbers) and found that the yellow and red totally over nucleated my crystalline glaze, but the brilliant red did not. See http://www.puttgarden.com/crystal/2007/6-18-07/DSC05489.jpg I have performed a series of tests where I varied the zinc oxide, silica and frit content of the glaze and was able to influence the nucleation rate. See http://www.puttgarden.com/crystal/2007/12-3-07/DSC07799.jpg I have prepared new slips using these 3 plus 2 others and combinations of them. See http://www.puttgarden.com/crystal/2007/12-4-07/DSC07832.jpg. I planned to glaze test tiles of each stain with my normal glaze plus one with 2.5% and 5% less zinc oxide to help hone in on the composition which will produce a balance of crystals and background?
(I apologize for the bombardment of questions.)
Phil Hamling
PS John, I can bring a series of these test tiles with various stains, plus the left over mixed slips I have to the workshop. Any interest?
Phil Hamling
376 County Route 1
Warwick, NY, USA 10990