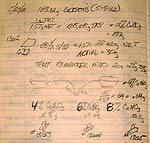I set this page up to facilitate collaboration between people who have the
ability to contribute and benefit from shared ideas, trials and results. those
listed below have access to this page. The main rule is that you share all.
This is the e-mail I sent to everyone.
|
Joerg Baumoeller |
Gordon Czop |
John Dunlavy |
John Tilton |
10-2-12 Joerg's thoughts on Rita Bassett's article
Moybdan_essentials.doc
7-26-12 No titania!!!!

|
|
Gordon's
Base |
Mixture #1 |
Mixture #2 |
Add to 100
Base Solids |
Add to 100
Mixture #1
Solids |
|
F3134 |
31.72 |
|
|
|
Lead Bisilicate |
4.53 |
|
|
|
Whiting |
18.13 |
|
|
|
Silica |
12.39 |
|
|
|
EPK |
18.13 |
|
|
|
Add 50% water |
|
|
|
| Calcium Molybdate |
|
4 |
|
| Black Copper Oxide |
|
1.5 |
|
| Cobalt Carbonate |
|
.75 |
|
| Manganese Dioxide |
|
.75 |
|
|
Gerstley Borate |
|
|
5 |
|
|
|
|
|
| Glaze Load (g/sq.in.) |
1.0 |
|
|
| 1080C Hold (hr.) |
1.5 |
|
|
|
| |
|
|
Mass (g) |
 |
Gordon's Base |
|
100 |
| F3134 |
|
5 |
| Calcium Molybdate |
|
4 |
| Rutile |
|
2 |
| Black Copper Oxide |
|
1.5 |
| Cobalt Carbonate |
|
0.75 |
| Manganese Dioxide |
|
0.75 |
| Glaze Load (g/sq.in.) |
|
0.5 |
| 1080C Hold (hr.) |
|
4 |
7-22-12 I just took these out this afternoon after firing with a 4
hour hold. The pieces with 6% and 8% rutile are bizarre and don't fit the table.
I'll wait until the 1 hour and 0 hour hold firings are finished before putting
up photos. I would swear I screwed up the formulas except I made the first one
shown below of the same materials.
|
|
 |
|
|
|
|
No rutile, less: copper, cobalt, manganese & iron plus 1/2% tin oxide. |
|
|
I sent out an e-mail
to the group showing the results of my test from 7-18 on 7-19 and later the
photos shown below under 7-20-12. I received comments as follows:
Gordon Czop,
Joerg Baumoeller,
John Tilton.
The basic explanation is I batched the
following, which I have been referring to as Gordon's Base. For a detailed explanation of compositions see
"gordon's glaze analysis 7-21-12.xls"
|
Mass (g) |
| F3134 |
210 |
| Lead Bisilicate |
30 |
| Calcium Carbonate |
120 |
| Silica |
182 |
| EPK |
120 |
Then mixed the ingredients in a blender with 340 ml water
and 1 drop concentrated Epsom salt solution. (The ES
functions a de-flocculent in these small concentrations as opposed to as a
flocculent when added in the 1 to 2% range.)
|
To a 151g sample of this base glaze, which
contains 100g of solids, I added the following
colorants. |
| Sample Name |
|
G1 |
4% Rutile |
|
G2 |
0% Rutile |
|
|
Rate |
Mass (g) |
|
Rate |
Mass (g) |
| Black Copper
Oxide |
|
1.5% |
1.5 |
|
1.5% |
1.5 |
| Cobalt
Carbonate |
|
0.75% |
0.75 |
|
0.75% |
0.75 |
| Manganese
Dioxide |
|
0.75% |
0.75 |
|
0.75% |
0.75 |
| Calcium
Molybdate |
|
4.0% |
4 |
|
4.0% |
4 |
| Rutile |
|
4.0% |
4 |
|
0.0% |
0 |
|
|
|
|
|
|
|
|
then blended equal amounts of G1 and G2 to get
what I called G1/2 or 2% rutile |
|
|
I applied these glazes to 4" square tiles at an ~1/2g
/ sq.in. loading. The "math" goes something like John cited ".....
when you calculate the amount of dry glaze that should go on a tile, the
calculation is 16 x .5 x 1.51 = 12.1 grams of glaze per tile." I targeted 12g
per tile. For each tile I placed it on a scale and zeroed it. Then I put the
tile on a banding wheel and applied an even coating until I got to 12 grams. I
kept track of my progress by periodically putting it back on the scale. I was
able to hold the loading in the range of 12 to 12.5 grams in most case.
I prepared 3 tiles of each formulation at a 1/2 g/sq.in loading as well as one
of each formulation at a 3/4 g/sq.in loading.
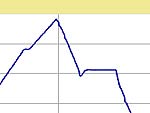
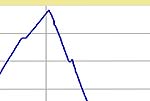
I fired them using the same schedule except I varied the 1080C hold time from 4
to 1 to 0 hours. The basic schedule and typical firing curves are shown below.
| Firing Schedule:
Segment,
Ramp (C/hr), Temp (C), Hold (min.) |
4 Hour
Hold |
1 Hour
Hold |
0 Hour
Hold |
| Segment |
Rate (C / min.) |
Temp. (C) |
Hold (min.) |
 |
 |
 |
| 1 |
200 |
1171 |
10 |
| 2 |
150 |
1305 |
0 |
| 3 |
340 |
1050 |
0 |
| 4 |
240 |
1080 |
240 |
7-20-12 Effects of titania content and hold
time.
7-18-12 Gordon's glaze - 4 hour hold.
7-15-12 Gordon's glaze with 1.5% black copper
oxide, .75% cobalt carbonate and .75% manganese dioxide and varying rutile
content applied at a rate of 1/2 g/sq.in.
Firing Schedule:
Ramp (C/hr), Temp (C), Hold (min.)
200, 1171,10
150,1305, 0
340, 1050, 0
240, 1080, 240 |
 |
 |
 |
|
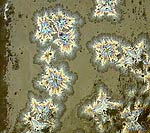
Revisiting one of Herbert Sanders' ^4 recipes
and trying to get the moly content right. |
|
|
|
 |
 |
 |
 |
|
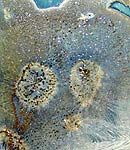
The crystals and iridescence are almost
impossible to see unless in reflected white
light |
This is the first time I've seen any crystals
come from one of Sanders' recipes. At this glaze
loading 3% MoOx seems to be the
trick. |
|
It looks like 4 1/2% red copper oxide, 1 1/2% cobalt carbonate and 1 1/2%
manganese dioxide is more than this wimpy ^4 glaze can digest. |
 |
 |
 |
This
test has samples with a line blend of from 0 to
4% molybdenum oxide |
5-26-12
|
From Joerg......Phil...
did you fire these with the "Gordon's CaMoO3
glaze" 5/10/2012? |
|
F3134 |
|
27.70 |
|
Lead
Monosilicate |
3.96 |
|
Whiting |
|
15.83 |
|
Silica |
|
24.01 |
|
Ball Clay (OM4) |
15.83 |
|
Rutile |
|
3.17 |
|
Powellite |
|
5.07 |
|
BCuOx |
|
1.58 |
|
CoCO3 |
|
2.86 |
|
|
Total |
100.00 |
|
Reply from Phil.......Yes. After
recalculating to 100% with all the additions the
recipe used was as appears on the left: |
5-23-12
 |
 |
 |
 |
|
I thought I was going to fire this without the 4 hour hold, but screwed up.
It went through with the hold. |
I added 3% cobalt carbonate to Gordon's glaze |
The cobalt resulted in smaller crystals much like it does in zinc silicate
crystalline glazes. |
Psycho |
 |
 |
 |
 |
|
Line blend containing 4%, 6% and 8% powellite. |
4% powellite. |
6% powellite. |
8% powellite. |
 |
 |
|
 |
|
It would be nice to get these to where they are a little less blah and speak
to the viewer from across the room. |
|
|
|
5-22-12
|
From Gordon......:The process for
roasting MoS2 to MoO3 is done the same way
(without CaCO3) as synthesizing powellite.The
sparkles you see on the plate of powellite are
probably some MoO3 (looks like needles) that
sublimed and recondensed on the surface, see
photo of roasted MoS2>MoO3. |
 |
John..On Phil's blog, a while
ago, there was a picture of MoO3 and WO3 that
Gordon had roasted. Would this have been done in
the same process as roasting the Powellite, but
without the Whiting? Can you just put MoS2 and
WS2 into the plate, fire it up in the same way,
and end up with MoO3 and WO3? |
 |
From Gordon......:Same firing
schedule and glaze constituents except as noted.
The dome piece was fired in a saggar and the 100
gram cup in the open, 4 hr hold, Third
firing for the dome:1st: 3% Powellite10%
Manganese Carb and 2% red CuOx. One crystal in
the drippings on underside of piece. 2nd: 5%
powellite. Completely covered with a sheet. 3rd:
4% powellite, 2% Manganese Carb and 1% red FeOx.
Cup: glaze same as 3rd fired dome, 0.6 g/si
Gordon |
 |
From Jon Dunlavy....try with 6% titanium diox, 4% Moly
triox. I color most of my tests with about 4% cobalt ox. Frit 3278 is also worth
testing.....in response to John Tilton writing ..Now I need to decide how to
spike them up so that there is a chance for some Mo Crystals. My thought is to
add 4% Molybdenum and 4% Rutile and a bit of color, say less than .5% CoCO3.
Just enough for some color, a nice light blue.
Does this seem intelligent for a start?
 |
From Ulrike......Phil, bismuth is
strong flux, even more than
lead.....Theoretically you could use it to
substitute the lead completely.
Bismuth is the flux part of lustre overglazes,
Feri surely will use it. And as lustres are
reduced, this surely will work. But
for me it is interesting in this context,
because it also reacts with molybdenum and
crystallizes in the tetragonal system.
But the two ......are
details from the tall bottle.
Two glazes overlapping, the black one with lead
and no Bi2O3, flowing over a white one, no
coloring oxides added, with bismuth.
Bismuth crystals seem to be smaller - but I
guess they just need another firing cycle ...Maybe
they crystallize best at another
temperature/holding point. |
 |
5-21-12
e-mail with thoughts from John Dunlavy.
US Patent 2663658, Ornamental Crystalline Glaze
From John Tilton
5-19-12 I came up with some
recipes to test based on Ulrike's formulae using GlazeMaster software. This
was kind of frustrating at first because it did not generate the exact same
numbers as did the Online Glaze calculator. I recalculated Gordon's 3134 recipe
with it and started from there.
5-18-12
e-mail reply
to
John Tilton's "Swimming out to the Ship" with thoughts from John Dunlavy.
e-mail reply to
John Tilton's Twisted Crystals with thoughts and suggested formulae from Ulrike.
 |
In it she wrote "
One more thing to the Koerner: I have an article
written by prof. Ralf Busz, where he is
mentioned. mentioned in a context of mo glazes
with Bismuth - not B2O3. Therefore I
thought the B2O3 would be just a printing error
and calculated the recipe without it. Bismuth is
very interesting in these glazes, I found." |
Metallurgy For
Dummies - Bismuth Alloys
Twisted Crystals e-mail from John Tilton.
5-16-12 In this test I took the glaze from 5-11-12 (copper and
rutile) and added 1/2% each of cobalt carbonate and manganese dioxide, then used
the same firing schedule.
 |
 |
 |
 |
|
I added extra powellite by eye ("EPBE") (~10%???) to the last bit of glaze
and applied a band to the top of a previously glazed vertical tile where an
1/2 g/sq.in. loading produced no crystals. |
It was nice to see this in the bottom of the kiln. |
This tile with glaze loaded at 1/2 g/sq.in. produced a crystal / background
ratio very similar to to the initial trial without cobalt. |
|
 |
 |
 |
 |
|
All the crystals from the last 2 firings seem like the crystals formed then
started to break apart. I wonder if they formed during the initial descent
from top temperature then were changed during the "hold". It looks like the
lattice has shifted (grown in one direction and shrank in the other)
splitting them apart. |
The 4" tile on top was a refire of a piece previously glazed with "P2A" (see
note below) with EPBE added at a rate of ~ .2 g/sq.in. |

The 4" tile refire, previously glazed with "P2A" with EPBE added was
completely covered with crystals on the half originally loaded with 1/2 g/sq.in.
P2A and less densely covered with ~1/8" square crystals on the half originally
loaded with 1 g/sq.in.
"P2A" was made of the formula I show on 4-22-12 where I commented "more
closely match Pukall's unity formula numbers using materials I have" with 2%
molybdenum oxide added. It produced no crystals during the first firing
whether applied at loadings of 1/2 or 1g/sq.in.
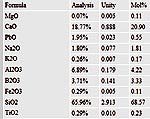
Its
formula is shown below.
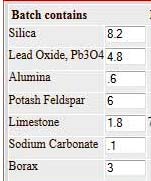
I found these 2 abstracts online via Google this morning. It
looks like they contain some very useful information.
5-15-12 Joerg....30 years for Bayer, but i am not a chemist...
I think your dark black powder is probably
molybdenum disulfide
Photos from Joerg
|

|
 |
|
Remarks: |
|
|
|
|
|
Consistence
Glaze :
liquid-creamy |
|
|
brushed : middle
thick, 1-2 mm |
|
|
|
all on
porcelain
plates Vicente
Diez, Spain, |
|
bisqued
at 1100°C , 10
cm x
15 cm x
0,5 cm |
|
 |
|
“S7” = “MA #4 Glaze” cone 9, page 44 Sanders book.
(Hommel Frit
replaced with Frit 3110).
|
“S4” = “MA #1 Glaze” cone 9 , page 43 Sanders book
|
|
FS S4 + S 7 |
|
|
in 7,5 h to
1260° C, 15 min
hold |
|
in 1 h to 1110°
C, 5 h hold |
|
in 1 h to 1060°
C, 1 h hold |
|
"End" |
|
|
|
|
 |
 |
 |
 |
 |
 |
|
|
Out of 11 formula proposals from Ulrike “3”,
“4”, “6”
showed (tiny...) crystals in my
kiln...
|
K/1 |
K/2 |
|
|
|
FS: |
|
|
|
|
|
|
|
|
|
Lead
Mono Silicate
|
45,7 |
44,2 |
|
|
|
in 6 h to 1250° C, 20 Min hold |
|
|
|
|
|
|
| Frit M1233
|
21,9 |
21,2 |
|
|
|
in 35 Min down to 1150°C , 10 Min hold |
|
|
|
|
|
|
potash feldspar
|
5,6 |
5,4 |
|
|
|
in 5 h down to 750°C |
|
|
|
|
|
|
| Caolin
|
3,9 |
3,8 |
|
|
|
End |
|
|
|
|
|
|
|
|
| Whiting |
9,1 |
8,8 |
|
|
|
|
|
|
|
|
|
|
|
|
| Flint
|
10,8 |
10,4 |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Consistence Glaze : liquid-creamy |
|
|
|
|
|
| 3.) K/1 with 4%
black Molybdene oxide
(moly disulfide??) |
|
brushed : middle thick, 1-2 mm |
|
|
|
|
|
|
| 4.) K/1 with 4%
black Molybdene oxide
(moly disulfide??) + 4%
Bi2O3 |
all on
porcelain plates Vicente Diez,
Spain, bisqued at 1100°C , 10 cm x
15 cm x 0,5 cm |
| 6.) K/2 with 4%
black Molybdene oxide
(moly disulfide??) + 4%
Bi2O3 |
|
|
|
|
|
|
|
|
|
Phil : Thanks for the notification.
Just chiming in to agree powellite is not absolutely
necessary and cone five and hotter is effective.
(Synthesizing powellite from MoS2 evolves SO2 and/or SO3,
very nasty) Gordon
John.....could
make sheelite (calcium tungstate) by the same roasting process as with
powellite?
|
5-12-12 |
Short Wave UV |
|
|
 |
 |
|
|
|
Left to right: 1g / sq.in. and 1/2 g /
sq.in. glaze loading. |
|
|
|
|
|
Short Wave UV |
|
Short Wave UV |
 |
 |
 |
 |
|
|
|
|
|
5-11-12 It appears that "holds" are not needed in the firing
schedule to promote the growth of Powellite crystals.
 |
 |
 |
 |
|
The piece, by Gordon, on the right, fired in a
saggar, suggest a slow cooling, helps grow
larger crystals, These used the formula and
firing schedule, listed below. |
The pair on the right is colored with 2% MnO2
and were fired using the previous schedule with
only a 1 hour hold. The saggar fired piece has
the largest crystals yet, It weighed a little
more than it's counterpart on the left,
suggesting further that a slower cooling from
the top temperature promoted the growth of
larger crystals. |
 |
 |
 |
|
|
The 6" square tile was fired on the floor on top
of a piece of ceramic fiber insulation. |
These crystals seem to crack apart as they form. |
|
|
 |
 |
 |
 |
|
I made a series of vertical tiles with 3/4, 1
and 1 1/2 g / sq. in. loadings. The first and
last formed crystals on one side only. The 1 g /
sq.in. loading formed virtually none. |
|
I believe the crystals grew larger partly
because it was insulated and cooled more slowly. |
Powellite, Black Copper Oxide and Rutile. |
|
|
|
|
|
|
|
|
|
|
 |
Firing Schedule:
Ramp (C/hr), Temp (C), Hold (min.)
200, 1171,10
150,1305, 0
340, 1050, 0
240, 1080, 240 |
 |
 |
|
This formula had 4% rutile, 4% powellite and 2%
black copper oxide added. |
|
Results |
|
4-29-12 Fuming
Fuming 5-4-12 Bill Campbell wrote "Did you
dissolve your SNCl in alcohol and water? If that is sprayed into the kiln, and
not directly on the pieces, you should be able to watch the luster develop.If
you have an old piece that has silver in the glaze, try that. The tin really
likes silver, and even better the combination of silver, and cobalt. A little
reduction after the fuming helps.
4-28-12 Making Powellite
I mixed molybdenum trioxide (MoO3) and
whiting (CaCO3), screened them 2X, then fired to 800C and held for 1 hour to
produce powellite (CaMoO4). I ground it in a mortar and pestle then passed it
through a sieve.
Gordon roasted
100g CaCo3 +160g molybdenum disulfide at 760C.
It still showed some signs of sulfur, but can be stirred and reheated to
complete the reaction. See 2-4-12.
|
|
|
|
|
 |
 |
 |
 |
| MoO3 -
CaCO3 (right) converted to CaMoO4 (center) |
Powellite
fluoresces under short wave UV light in crystals
on pottery as well as in powder form. |
A Herbert Sanders recipe with 4% and 8% MoO3
added. |
The iridescent areas and "egg yoke" colored eruptions of the Herbert Sanders
samples both fluoresce yellow indicating they are powellite. I think the next
step would be to reduce the moly content.
Gordon and I spent the afternoon discussing molybden glazes and comapring reslts.
He later wrote: Phil, Bassett says two or three times
that the crystals flow with the glaze and collect in areas.Gordon
4-27-12 Pawellite
Powellite
4-25-12 Phil, The translation was helpful. Thanks,
Gordon
(I sent Gordon this section from
"My experiences with crystal glazes / part two", Dr. W. Pukall,
Sprechsaal - Nr. 37 / 1908
Translation
Ulrike Franck)
 |
new over old plate fluid glaze . |
 |
stiff
glaze from article on today's pot
didn't work no crystals
on neck put over glaze wit powelite. 40 -3134,
20 ea (wollastonite, epk + silca) + 3 -5%
powellite
powellite
100g caco3 160g moly disulfide
make powellite, put it in the glaze, has to be
a glossy shiny glaze
crystalline glaze firing |
_________________________________________________________________________________________
4-25-12
Development and control of
molybdenum crystals in a stoneware glaze,
Bassett, Dorothy Rita 1949-, 1978 MFA Thesis, Universtity of Florida This is
a 7MB file
Note pg 47 for discussion regarding fuming to enhance and intensify iridescent
qualities of molybdenum crystals
_________________________________________________________________________________________
4-22-12 Analysis of
molybdenum glaze recipes
4-12-12 Gordon's
thoughts on molybdenum glazes. Phil, I hope these notes are helpful, my
experience, attempted to be
presented in some coherent, useful fashion.Your thoughts on improving it in any
way are welcome. Thanks, Gordon
4-11-12 E-mail with Ulrike
Intro and reference to technical article by Koerner.

Complete Book: Transactions of the American Ceramic Society Volume 10
3-18-12 "Molybdenum" crystals are illusive to
say the least. Plus anyone with a clue has
zipped lips.
|
Gordon grew these crystals from a pellet of
powellite placed in the closed space between 2
plates. |
 |
 |
 |
|
|
|
 |
 |
|
 |
| UV suggests they are calcium molybdate crystals. |
|
.A
Herbert Sanders recipe with 4% MoO3 added. |
2-19-12 Bulldog Pottery.....Iron red molybdenum crystalline

2-13-12
Gordon, The test results of the 10% MoO3 and WoO3 in
crystalline glaze and liner glaze was bizarre. The liner, which really never
runs, reacted and ran off the tiles. MoO3 moved the most and made streamers down
the tile. The crystalline glaze, which always runs, didn't move a bit. I'll try
and get some photos tomorrow. It's nap time now.
Phil
2-12-12
Gordon, This was after firing to 850C, 1 hour hold, cool
150C/hr to 650C. I think I'm going to stop this one here. These crystals just
look happy growing on the surface. The other tiles with 10% MoO3 and WoO3 in
crystalline and non-crystalline glazes will be out tomorrow. I think I'll
look at loading a crystalline glaze (without zinc) with calcium carbonate and
MoSi2. Phil
2-11-12
Zinc Molybdate,
SiO2 - ZnO2 Phase Equilibria,
Calcium Molybdate,
Calcium Tungstate, information.
2-10-12
Phil: I ran an experiment with a high calcium glaze
and separately the components for CaOMoO3. Bottom line: nothing. Glaze 3134 37%,
epk and silica both 20%, whiting and wollastonite both 10%. Firing to 1160 and a
three hour slant soak to 1060. Next will try free MoO3 and the glaze (as
originally planned), perhaps some moly in the glaze on another piece as well.
Regarding those latest photos I'm glad that was glaze and not some alien
biological entity. Gordon
2-9-12 I repeated the previous tests using crystalline glazed tiles at up to
950C? and saw some deposition or crystallization on the surfaces. I did not want
to go so high in temperature as to start taking the zinc silicate crystals
apart. I failed to keep geed notes on the exact temperatures. I could have
stated exactly what they were during the time I was performing the tests
but abandoned the effort when it appeared this was not the correct path and
failed to record them.
Gordon, I did another test in which I put 2 pre-fired
crystalline glaze samples in the saggar with 1/2g MoO3 powder on each catcher.
After 1 hr. at 800C it seemed that only ~ 1/2 of the stuff went poof and I got
this.
2-7-12 Samples were fired in the saggar shown below. The thermocouple showed
that although there was an ~50C difference between the inside temperature and
the kiln temperature on heating the difference was minimal during holds in the
1000 to 1200C region. At 1000C with a 1 hour hold there some volatilization of
moly. I did not see much reaction with the glaze if any. After firing higher (to
1100) I saw the moly materials completely volatilize and a significant matting
of the glazed surfaces. There did not appear to be any deposition on the bare
porcelain surfaces or saggar walls. The glaze took on a bubbled appearance. I
surmise that moly got into the glaze at the lower temperatures, but at the
higher temperature (1200) began to form bubbles under the surface, but was
"frozen" in place upon cooling preventing it from boiling out.
Gordon,
After up to 1000C - hold 1 hr - cool to RT, then up to 1100C
- hold 1 hr - cool to RT and finally up to 1200C - hold 1 hr - cool it looks
like something got in the glaze that wants to get out in the worse way.
Phil
I don't remember if I
told you or not but I ordered 2 1/2 kg of
Molybdenum trioxide (molybdic anhydride) from this company. I got the attached
MSDS today. I am surprised by the
info in the Physical Properties section, specifically the melting point and
boiling point data.

2-6-12
Gordon., I fired my
test setup for 1 hr. at 1000C (1832F). All the moly oxide and metal vaporized –
went poof. I do not see any on the inside of the crucible, on refractories or on
porcelain. The bright shiny test pieces took on a matt appearance and seems to
have a shiny haze on it as if there are many, many little molybdenum trioxide
crystals on the surface. I sent it back up for 1 hr. at 1100C and
should see the results tonight. Phil
2-5-12
RE: Trying
to understand molybdenum crystals, e-mail dialogue with Halmos Ferenc.
Gordon wrote: Consider MoO3, it has
a documented vapor pressure curve. See attached spreadsheet from CRC handbook
data.
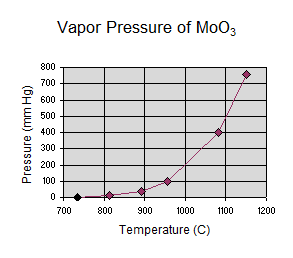
Gordon,
I have this setup in the kiln today. It will get to 1000C at
~ noon. Do you think I should fire it higher?
The MoO3 vapor pressure curve shows that it has all sublimed at ~1150C, but I
wonder if I really need to go that hot. Kind of like water evaporates pretty
significantly below 100C.
These photos show 2 different ^10 glazed tiles, with a fair
whiting content. I've added 1g MoO3 powder and a few square inches of scrap
molybdenum (metal) screen to the mix. I have covered the setup with an inverted
alumina crucible to serve as a saggar and included some alumina fiber paper as a
gasket. Also there is a thermocouple inside the saggar so I can get a handle on
the inside temperature as well.
What do you think?
Phil
258-2936
2-4-12 Re-firing test pieces with calcia containing glazes in an
attempt to fume molybdenum oxide vapors. An alumina crucible was used as a
saggar. Samples of molybdenum wire and molybdenum oxide were placed on the
catchers on the bottom.
2-4-12 Discussions with Jon
Dunlavy
"Molybdic
oxide crystallizes on the surface of the glaze and they are not grown like other
crystals with a slow cooling." "Molybdenum trioxide is the usual
source"
2-4-12 Some interesting information on water
crystals
2-4-12
Gordon,
Jon does have some interesting stuff going on here.
I plan to re-read Sanders' book today.
I was real interested to review the MoO3 vapor pressure
curve you supplied. I wonder how it compares to that of zinc oxide. Do you have
the CRC Handbook and the ability to look this up? I've Googled "zinc oxide vapor
pressure" and see there is a lot of information out there, but one would need to
buy a bunch of books or belong to a university to get to it.
I'd love to work with you to develop a molybdenum glaze. How
do we get started?
I batched up some of the material you gave me with a
crystalline glaze and whiting / Custer feldspar based one, at a 5% addition
level, to try getting started by using the "poke and hope" method. I'm not sure
where this will go.
Phil
______________________________________________________________________
Here's more food for thought
http://apollo.lsc.vsc.edu/~wintelsw/MET1010LOL/chapter07/
2-4-12
Phil:
Here are MoO3 crystals formed by roasting MoS2 at 760c. The
reaction is exothermic so initially the MoO3 is hotter, it sublimes & then cools
and condenses to form crystals.
They do not fluoresce;they are 3 dimensional needles. Am
close to being convinced the crystals folks grow in glazes are CaOMoO3 which
would fluoresce yellow.
If so perhaps the MoO3 condenses onto the glaze surface,
interacts with CaO in some form and
starts the crystal.
My next step will be to make a
cone 3-4 glaze with calcium( but no
lead) in it, fire it alone first, put it in a saggar with
MoO3 separate but enclosed, heat to 1150 c or so and then do a very slow cool to
about 1000c.
Thanks for the fascinating information on water/weather,
maybe I'm too easily convinced but it seems clear that something like that is
happening in the moly crystal glazes.
Gordon
Gordon,
Thanks for the info.
Today at work I heated some pieces of moly wire and sheet
slivers with a torch. They seemed to glow hotter than the surrounding refractory
and put out a thick yellow -white smoke. The reaction and glow stopped when I
removed the heat but the smoke kept coming for a little while. I think the risk
of explosion is minimal.
I brought home some alumina crucibles which I will use as
saggers and heat up some stuff. I'll photo the setup and any results worth the
effort.
Phil
| 1-31-12 |
|
 |
 |
| Does anyone know Jon
Dunlavy? I found this photo on Flickr after
searching for "Molybdenum Crystals" |
Here's another of his pieces
I found on someone else's Flickr account.
Then I found his website but didn't see any
molybdenum crystalline glazes.
http://jondunlavy.daportfolio.com/ |
2-20-2011
Moly crystals by Bulldog Pottery.


Back to Crystalline Glaze Information Page