8-2-12 .5% Black Copper Oxide, .05% Cobalt
Carbonate, .2% Red Iron Oxide and .1% Manganese Dioxide
7-28-12
7-28-12
7-22-12
 |
 |
|
Mo glaze
recipe found in Sprechsaal, vol 122,
No 4, 1989, written by Walter Kerstan. I do not know
how it works. |
7-20-12 Effects of titania content and hold time.
7-18-12 Gordon's glaze
7-17-12 This is the first time I've seen any crystals
come from one of Sanders' recipes. At this glaze loading 3% MoOx seems to be the
trick.
7-15-12 Revisiting one of Herbert Sanders' ^4 recipes
and trying to get the moly content right, plus looking at the influence
of rutile in another.
 |
 |
 |
 |
|
I need to get some real kiln furniture for this small kiln. This set up
includes reticulated alumina plates and sapphire bars. |
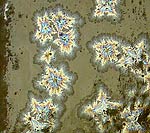
This test has samples with a line blend of from 0 to 4% molybdenum oxide. |
|
A different moly glaze with 1.5% black copper oxide, .75% cobalt carbonate
and .75% manganese dioxide and varying rutile content. |
7-13-12 Yesterday Phil and I made a quick stop at the
new building.
7-9-12
7-4-12 Feri has been making some tremendous work. It is
good to see him back at it again. He has to be in that group of 3 crystalline
artists.
 |
 |
 |
 |
|
One of my favorites |
Before and After |
A view from behind the scenes |
6-25-12 I made a mixture of ~100g tin chloride + 100g isopropyl
alcohol and used Jamie's booze bag to drip it in while I held the kiln at ~1200F
by adjusting the hi - med - low knobs. I had to add ~40g water to get all the
salt to dissolve. For the first 20 minutes it was going at ~ 1 drip per second,
then for the next 20 minutes ~5 drips per second.
 |
 |
 |
 |
|
It smoked pretty nastily - I guess putting out HCl |
but I don't think there was enough reduction to make much of a reaction. |
Pieces had a white dust all over them which wiped off easily. I guess it is
tin oxide. |
This silver containing piece seemed to change slightly and show some
iridescence.. |
 |
 |
 |
 |
The green copper piece at 9 o'clock did not change. The partially red,
previously reduced, copper piece at 11:30 seemed to re-oxidize and lost most of
the red color. I'll do it again at 1300F with much more alcohol.
 |
 |
 |
 |
|
I recently spotted these at "Art on the Green" |
Savannah and Kat |
I had to do some Photoshop work because Kat was wearing these ridiculous heels
which put her up in the stratosphere making Savannah look like a dwarf.
6-21-12
Andy wrote: "Wow Phil, Your photos look better than the
actual pots! It is amazing how a little bit of sunlight really gives that glaze
the spark of life. Technically raku is a noun. Proper English would
be to say "it was raku fired" but I know the words "I raku-ed it" have come out
of my mouth and quite a few other potter's mouths as well. Andy
6-20-12
 |
 |
 |
 |
|
Spring is one of my favorite time of the year in the yard. |
These are the last pallets of finished goods being moved from our old
warehouse into the new place. |
6-14-12
 |
 |
 |
 |
|
This is a good reason to bisque fire things. It helps minimize breakage in
transit. |
Stuff from Jamaica. |
6-1-12 We are going back to Jamaica for a while!
5-24-12
5-16-12
5-13-12 Maybe I'll just pack it in and concentrate on the bait and tackle
shop.
 |
 |
 |
 |
|
|
Molybdenum, disilicide heating element holders, Block fiber insulation and 2
sizes of finer insulated heaters. |
A sintering fixture for powdered metal components precision machined from an
alumina fiber composite board. Although pure white when fired in oxidation
materials like these, pre-fired in a hydrogen atmosphere, often take on a
grey to charcoal appearance. |
5-12-12
 |
 |
 |
 |
|
|
Short Wave UV |
|
Short Wave UV |
 |
 |
 |
 |
|
|
Short Wave UV |
|
Short Wave UV |
 |
 |
 |
 |
|
Natural Light + (a little) Short Wave UV |
Short Wave UV |
Natural Light then Short Wave UV |
|
 |
 |
 |
 |
|
|
Natural Light + Short Wave UV |
Short Wave UV |
|
 |
 |
 |
 |
|
|
Natural Light + Short Wave UV |
Short Wave UV |
|
5-11-12
5-8-12 We are
expanding at work. This new building will add an additional 50% to our operating
space.
 |
 |
Hopefully we can get
through this so I can move on to some studio time. I've only been able to
get 5 to 10 minutes at a time into designing and building my gradient kiln
for firing samples. It should help compress the crystal learning curve
tremendously. |
 |
|
I know Frank Lloyd
Wright didn't have a hand in its design. It's hard to believe we
started looking at this place 6 months ago and today is the big day -
closing. |
|
Phil took me fishing for my birthday on May 6. I was born 56 years ago on
5-6-56. All we caught were 6 sand sharks. This poor baby was "delivered"
(prematurely) on deck. |
5-3-12 The best tile was the one with the thickest, mud cracked
glaze application.
 |
 |
 |
Patti and I celebrated our 25th anniversary with dinner at the
Glenmere mansion tonight.
|
 |
 |
 |
 |
|
Now I just have to learn how to get these bigger and up out of the glaze
catchers. |
5-1-12 I finally pushed the START button again!
 |
 |
 |
 |
|
|
|
Reconstituting dried glaze was a PITA. I had no clue what the water content
was and the gelatinous effect of Veegum Cer was nowhere to be seen. The
glaze application of 4 coats shrank and cracked like a pig. |
 |
|
 |
 |
|
Fuming with tin chloride lightened the pieces slightly and added a fringe of
iridescence in the lowest temperature rings of copper, but gave essentially
unremarkable results. |
|
David and I gave Dad his final buyout check for the business. I can't
believe 10 years went by that fast. |
Cole got his first tom this morning out back with Phil. |
4-29-12 I read that fuming can enhance the iridescence of some
crystals. So I got the stuff ans set the old oil bomb unit up outside.
 |
 |
 |
 |
|
Tin chloride, iron chloride and barium nitrate seem to be pretty nasty
stuff. |
20g of tin chloride |
Before |
Afterwards I saw a slight discoloration of the ceramic fiber block supports. |
4-25-12 Mental note: Working on understanding
"molybdenum glazes" with a group of collaborators has taken more time than
expected.
 |
 |
 |
 |
|
A sad day
indeed. |
Powellite crystals |
4-28-12
I get a strange feeling from modifying other people's work. |
4-16-12
 |
 |
 |
 |
|
|
|
|
What were you thinking about 30+ years ago? |
4-14-12
 |
 |
 |
 |
|
How Wizardly! |
|
|
I guess he meant it when he said "I'll send you some tile to play with". |
|
|
|
|
|
 |
 |
 |
 |
|
...one of Marsha's unglazed pots... |
Alumina Hydrate and glue is the way to go. |
Flowering peach |
|
Anne Melvin wrote: "Hey Phil,
Here is a photo of one of Marsha's unglazed pots, picked at random off her
shelf. I used phimatrix.com to check out the proportions. One can also use the
French Curve tool and the basic curve of the pot fits exactly. Anne"
 |
 |
 |
 |
|
Pieces from the Ginny Conrow / Marsha Silverman Workshop |
4-6-12
 |
 |
 |
 |
|
F413 base and 5% yellow uranium oxide with (from left to right) 0, 1, 2, 3
and 4% titania (back row) plus 1% green nickel oxide (front row). |
They've started planting onions. |
It's very dry. I've never seen the irrigation going in April before. |
Ceramic Fiber Settling Test |
3-25-12
Ginny Conrow / Marsha Silverman Workshop, Seattle Washington
Workshop Index
|
3-28-12 |
3-26-12 |
3-25-12 |
3-24-12 |
 |
 |
 |
 |
|
Throwing / altering demonstration and pieces from the first kiln firing. |
A little talking plus a full day of glazing. |
Helped setup, saw some sights and the evening slideshows. |
Got to Seattle at noon and spent the time getting acquainted. |
3-20-12
3-18-12 "Molybdenum" crystals are illusive to say the least.
Plus anyone with a clue has zipped lips.
 |
 |
 |
 |
|
|
|
UV suggests they are not exclusively moly oxide. |
|
 |
 |
 |
 |
|
Drippy white glaze tests with 20, 15, 10, 5 & 0% zinc oxide additions. |
. |
David Turner delivered this 5 DVD set on Sunday. Now all I need to do is get
a DVD player and a free day to watch them |
Sushi Deluxe |
3-16-12 Speaking of fishin'..... we plan to go for
sturgeon on
the Frasier River near Holly's, after Ginny's workshop and a trip to Bill
Boyd's. I've got my license and sturgeon tag all ready to go.
 |
 |
 |
 |
|
Bill Campbell |
The Wizard |
Still cleaning up after Irene. |
3-10-12 My fishin' buddy Bill sent me a bisque platter and
some fired pieces. If I want to grow up to be just like the best I see
there's a long way to go.
Metallic Glaze Recipes
3-7-12 Still working with F413 glaze with red copper oxide,
manganese dioxide and cobalt carbonate additions - with a few variations.
 |
 |
 |
 |
|
A new clay body with glaze containing my batch of F413 (2 on left) and one
of the Wizard's F413 batches (after adjustments to get similar background)
on the right. His 413 glaze comes out much darker than mine on that clay. |
I like the eyebrows. |
With tin - indium oxide added.
|
With neodymium oxide oxide added.
|
 |
 |
 |
 |
|
With 11% red copper oxide and 10% manganese dioxide added viewed in 2
different lights. |
3-4-12
 |
 |
 |
 |
|
|
. |
Line blends in this area should tell a lot. |
Drippy white glaze tests with 20, 15, 10, 5 & 0% zinc oxide additions. |
3-3-12
 |
 |
 |
 |
|
Finally......finally.....glazing some older work. |
Cuprous oxide (red) does not like to stay in the glaze. |
Test pucks of a high chromium castable alumina refractory. |
My Toyota 4 Runner turned 100K the other night. |
 |
 |
 |
 |
|
It has been an unusual winter with "warm" weather and little snow. |
I think a late fall application of Quicksilver for moss control is
responsible for the odd blotchy coloration on the greens this year. |
The moss sure has been loving it on the rocks this winter. |
|
|
2-27-12 |
2-22-12 |
|
|
 |
 |
 |
 |
|
I call this one "Clueless" |
Pictures of
man made crystals by SurfaceNet. |
Thermal Technology, llc - Crystal Growth Technology
web page |
2-19-12
 |
 |
 |
 |
|
Avi's laps are also good for cleaning up the
bottom of bubble alumina cylinders, like this one which will be on its way to a
customer that grows industrial crystals. |
Bulldog Pottery.....Iron red molybdenum crystalline |
Too much something and not enough background? |
2-18-12
Anhydrous Molybdates and Tungstates
2-15-12
 |
 |
 |
|
|
Did he call this glaze "Sunrise Blue" |
Or "Morning Blue"? |
AFU has a nice foamy kind of look to it. |
|
 |
 |
 |
 |
|
Uranium oxide engobe in carvings covered with clear F413 base glaze (left)
and F413 base +1% green nickel oxide (right). |
Left: F413 base with10% yellow uranium oxide + 1% green nickel oxide. Right:
F413 base with 1% green nickel oxide. Both on uranium oxide engobe. |
Clear F413 base glaze on (L to R) yellow, orange and black urania engobes. |
Who said bikers are stupid? |
http://prometheanpottery.wordpress.com/glaze-glossary-and-resource/glossary-of-glaze-colorants/
2-12-12
Zinc Molybdate,
SiO2 - ZnO2 Phase
Equilibria,
Calcium Molybdate,
Calcium
Tungstate, information.
2-11-12
2-10-12
 |
 |
 |
 |
|
2 Blue - New |
|
|
|
 |
 |
 |
 |
|
L to R: "bad" frit, 50:50 mix, "good" frit. |
|
|
A friend recently took delivery of a new batch of frit that plain old gave
crappy results. It's just not the same. Glazes made with it come out over
nucleated. It just didn't melt the same or grow crystals the same. The
manufacturer says it matches their specifications but it just didn't work.
Eventually a fix was found (I think by increasing the amount of it in the
recipe, lowering the zinc and possibly tweaking the silica content). This
formula adjustment process, I'm sure, consumed a bunch of time and money and
caused many, many problems including agita.
I know this was after the fact but I ran a test to confirm the stuff was "bad".
The 3 identical pieces above were thrown by The Wizard. They are made of the
same clay and are the same size and mass. Triplets so to speak. I mixed a batch
of this latest blue glaze using this problem frit and one with the frit I have
been using, plus a 50:50 blend of the two. I applied equal amounts (weighed to
within a gram of each other) to each piece and fired them side by side. I feel
the results were conclusive and showed yes indeed the new stuff is crappy.
2-9-12
2-7-12
2-6-12 Andy Boy came to play yesterday. It was Superbowl
Sunday so we didn't get it all done. He came back today to finish up the last
coats.
 |
 |
 |
 |
|
This piece has some nice carving in it. |
|
|
When I fist met him I couldn't remember his name. Then I found some help in
the refrigerator. |
2-6-12 NY Giants Superbowl Champs
 |
 |
 |
 |
|
The fans were chomping at the bit for a Giants' victory. |
Eli Manning was coaching Madonna during the halftime show |
Bradshaw showed us how easy it was to win by just plopping your butt down
over the goal line. |
In the end it's all about taking the Lombardi Trophy home. |
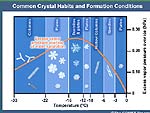
2-4-12 Some interesting information on water
crystals
 |
 |
 |
 |
|
|
Ice formed overnight on a car. |
Here's a combo where the crystals fluoresce yellow. |
1-31-12 After posting the question "Does anyone know Jon Dunlavy?"
on The Crystalline Glaze Forum Greg Beckman (mohawkpiper) replied "phil, i
know jon dunlavy. he works at the local clay store (clay planet) on
saturdays and the rest of the week works as some sort of chemist working
with glazes. ......... that piece you posted looks just like his work. G"
|
1-31-12 |
|
|
1-30-12 |
 |
 |
 |
 |
|
Does anyone know Jon Dunlavy? I found this photo on Flickr after searching
for "Molybdenum Crystals" |
Here's another of his pieces I found on someone else's Flickr account.
Then I found his website but didn't see any molybdenum crystalline glazes.
http://jondunlavy.daportfolio.com/ |
Today, after everything had cooled down, the olive drab from the original
yellow uranium oxide colorant is back. The most curious thing is that the
original engobe
sample, which was sitting wet for ~2 months, turned a bright
yellow color after bisque - which is what I expected to see in the first
place. |
@450C the sample using the original engobe, which appears yellow in the
photo at the left, seemed to be an orange red color.
What a tweaky, fickle trail this seems to be! |
xxxx
|
1-29-12 |
|
1-28-12 pm |
|
 |
 |
 |
 |
|
Then I thought maybe my notes were flawed and I actually mixed them at a 20%
level. While adding more powder I realized the yellow material I used in the
second and third trial was from a different container than from the
first. I went back to the original stuff for this trial and made one trial
using the original engobe. |
Although the 2 preparations looked different before bisque, afterwards the
difference were unremarkable and still no olive drab. |
I remembered that the first time I mixed the engobes by hand and passed them
through an 80 mesh sieve . The next time I blended just the uranium oxide in
water first and passed it through an 100 mesh sieve (Y1 thru B1). Maybe
particle size was the difference - so I went back to the original method (Y2
thru B2). |
 |
 |
 |
 |
|
Patti and I went to see Andy Boswell at an exhibition in a gallery in
Princeton, NJ. He had some very nice work there including some celadon green
and blue glazed pieces. |
1-28-12 am My plan was
"Today I'm
going to go back to exactly the same clay combinations and see if I can
reproduce my original results". Then I realized I had already tried those
combinations and got olive drab so it must be something else ....... but what?
1-27-12 Jamie wrote "I’d
say the (base clay) is definitely the difference, even if the engobe is the
same. I notice differences in results when the body is different, even if it has
the same engobe...."
1-24-12 I've been working with uranium oxide engobes
and are perplexed by the results compared to my last attempt. I was
expecting, after bisque, that the yellow engobe would turn to olive drab (as
it did before) and the black to stay black. Neither happened. Although after
bisque there were subtle differences there was no olive drab and no black.
They just tended towards looking more like each other, in 2 separate kilns.
I'm not sure what happened but would like to get a handle on it. This is one
of the rare times I feel like I am unable to reproduce previous results.
 |
 |
 |
 |
|
Before |
After |
Before |
After |
 |
 |
 |
 |
|
Previous experience from 11-25-11, left to right, 10% yellow uranium oxide
engobe covered pieces turned to olive drab after bisque firing. |
Left to Right: 10% yellow, 10% orange and 10% black uranium oxide engobes on
some of The Wizard's pieces. |
Left to Right: 10% black, 10% orange and 10% yellow uranium oxide engobes on
vertical test tiles. |
 |
 |
 |
|
|
Some innies and an outtie. |
Engobe placed in surface incisions. |
|
|
|
|
|
 |
 |
|
|
|
New bottles by Glenn Woods
(I have to give him a real camera for Christmas.) |
And one by Marsha Silverman who I can't wait to see at Ginny's in March. |
1-21-12
 |
 |
 |
 |
|
I'm looking forward to this piece from Matt Horne. |
Joerg Baumoeller and Peter
Ilsley in Joerg's studio. Photo courtesy of Josť Mariscal. |
An unique blue glaze by Paul Brown. |
Another interesting number. |
1-16-12
 |
 |
 |
 |
|
This stinker took 5 firings. |
A post fire reduced piece of Gordon's. I wonder if the deeper gold crystals
and pink background are from silver flashing from other pieces or if he
added some magic ingredients in his glaze. |
|
|
 |
 |
 |
 |
|
|
Now just to get this to stay on the pot! |
|
|
1-13-12
 |
 |
 |
 |
|
With the lid shut the Geiger Counter barely ticks. |
|
Feri pointed me to this book on luster glazes |
Yellow, orange and black urania powders before (bottom) and after (top) a
^11 oxidizing crystalline glaze firing. |
 |
 |
 |
 |
|
|
|
|
|
1-8-12
 |
 |
 |
 |
|
Bailey has a nice new SS barreled extruder which I set up on a quick
disconnect mount. |
Fabricating a lead lined ammo can, with copper foil liner, for storing
uranium oxide powders. |
1-2-12
Back to Crystalline Glaze Information Page


























































.jpg)